![]()
Small on the outside, big on the inside: Porous Organic Polymers
Author: Hakan Bildirir
Polymeric materials (i.e. plastics) are essential part of our daily lives in various forms from disposable tableware for our picnic to car parts for our mobilization. A step forward from the basic “end-user” products, polymeric compounds became interesting for electronic devices since the final materials are easier to shape (flexible) and structurally tunable for the aimed application with respect to the traditional inorganic counterparts. Moreover, polymeric materials have less geographical limitations when compared to inorganics; for example, reachability of inorganic electrodes used for Li-ion batteries are in control of the corresponding mining regions, however, organic polymeric electrode materials can be synthesized in anywhere of the world from the basic starting compounds.
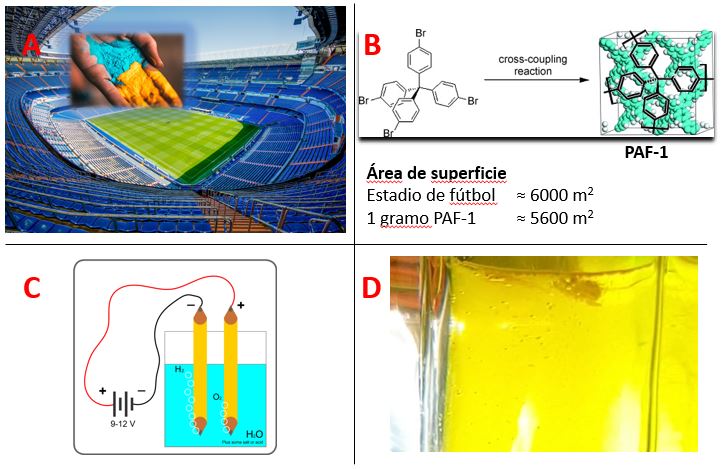
Aside from the abovementioned traditional polymers, which are soluble and linear form, a novel type polymer becoming more and more attractive for each passing day. Those compounds are highly crosslinked and rigid materials named “porous polymers”, which offer high surface areas along their (hydro)thermally stable backbones; one gram of those compounds can have an accessible surface area as big as a football field (Figure 1 A-B).[1]
Figure 1: A) Illustration of comparison of 1-gram porous polymer to a football stadium (images from pixabay.com). B) Chemical structure of a high surface area porous polymer and information related the surface areas (reproduced from ref[1] with the permission from Wiley-VCH, Copyright 2009). C) Basic electrolysis of water (Picture from Wikipedia). D) A porous polymer dispersed in water producing H2 gas (bubbles) via photocatalysis (picture captured from the video Ref[2]).
The high surface area of those compounds makes them very effective gas storage applications and catalytic uses. The empty voids (pores) of those compounds are ideal spaces to store gases, which makes them interesting for various applications such as fuel tank filling materials for hydrogen cars for safer, high capacity hydrogen storage.[3] On the other hand, the high surface area leads to more interaction points than non-porous analogues with the target compounds, hence the success of porous polymers for catalytic applications.
Speaking of hydrogen and catalysis together, porous polymers are probably one of the most promising catalysts for photocatalytic hydrogen production from water.[4] This is very similar to the experiment which we did in high school, electrolysis of water, but this time not spending electricity and only using some beams of light (Figure 1C and 1D).
Energy and environmental applications of porous polymers related to the combination of gas capture and catalysis is not limited only for hydrogen production from water, but also works for carbon dioxide (CO2) conversion and nitrogen fixation.[5] Former application is interesting to reduce the pollutant CO2 from the atmosphere whereas the latter is a green alternative to the traditional ammonia production procedure (Haber-Bosch process) which result in high amounts of energy consumption and CO2 pollution.[6] Moreover, aside from the standard photocatalytic applications, the prospective developments of the porous polymers are related to their integration to photo-electrochemical cells and organic electronic devices.[7,8] Such compounds are expected to demonstrate very interesting performances not only as a result of their high surface area and good stability, but also due to their high dimensional, electron-rich backbone offering advanced electronic conductivity.
At the photoactivated processes unit of IMDEA Energy, we develop porous polymeric photo(electro)catalysts to produce hydrogen from water, convert CO2 to useful chemicals, and produce ammonia like compounds through nitrogen fixation. Our goal is to keep the world clean and safe by finding solutions for producing sustainable and green energy and reducing the pollution caused by the old-style energy consumption (i.e. fossil fuels). Indeed, we collaborate with regional, national and international researchers thought different research project coordinated by us such us FotoArt-CM, SOL-Future and HYSOLCHEM, respectively. So, our research moves on to an interdisciplinary level, and get support by many funding agencies such as Comunidad de Madrid (CM), Agencia Estatal de Investigación (AEI), and European Commission (EU).
References
[1] T. Ben, H. Ren, M. Shengqian, D. Cao, J. Lan, X. Jing, W. Wang, J. Xu, F. Deng, J. M. Simmons, S. Qiu, G. Zhu, Angew. Chemie – Int. Ed. 2009, 48, 9457–9460.
[2] M. Trunk, G. Li, H. Küçükkeçeci, D. Burmeister, M. Obermeier, B. T. Bonkano, M. Schwalbe, A. Thomas, M. Bojdys, ChemRxiv 2022, DOI doi.org/10.26434/chemrxiv-2021-q1b4b-v2.
[3] Z. Chen, K. O. Kirlikovali, K. B. Idrees, M. C. Wasson, O. K. Farha, Chem 2022, 8, 693–716.
[4] M. Barawi, L. Collado, M. Gomez-Mendoza, F. E. Oropeza, M. Liras, V. A. de la Peña O’Shea, Adv. Energy Mater. 2021, 11, 2101530.
[5] M. Liras, M. Barawi, V. A. de la Peña O’Shea, Chem. Soc. Rev. 2019, 48, 5454–5487.
[6] L. K. Boerner, Chem. Eng. News 2019, 97, 1–9.
[7] H. Bildirir, V. G. Gregoriou, A. Avgeropoulos, U. Scherf, C. L. Chochos, Mater. Horizons 2017, 4, 546–556.
[8] H. Bildirir, Crystals 2021, 11, 762.
Contact
Hakan Bildirir, Postdoctoral Researcher at IMDEA Energy.
Víctor A. de la Peña O´Shea, Principal Researcher of IMDEA-E group of FotoArt-CM and coordinator of the programme.